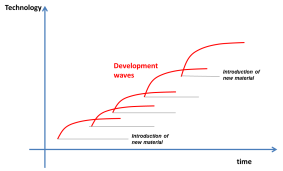
The discovery of graphene is leading to a new excitement in materials research. I have a notion that technology advances take place in step waves, where each step is both enabled and constrained by the materials available. Each time a new material (or material family is discovered), technology development starts very fast and then tapers off until another material comes along and ignites a new development wave.
Boron is Carbon’s neighbour in the periodic table and the discovery of graphene has ignited studies to see if a similar variation of boron would be possible.
Boron is a Group 13 element that has properties which are borderline between metals and non-metals (semimetallic). It is a semiconductor rather than a metallic conductor. Chemically it is closer to silicon than to aluminium, gallium, indium, and thallium. Crystalline boron is inert chemically and is resistant to attack by boiling HF or HCl. When finely divided it is attacked slowly by hot concentrated nitric acid.
Boron, Symbol: B, Atomic number: 5, Atomic weight: 10.811, solid at 298 K
“Boron has one fewer electron than carbon and as a result can’t form the honeycomb lattice that makes up graphene. For boron to form a single-atom layer, theorists suggested that the atoms must be arranged in a triangular lattice with hexagonal vacancies — holes — in the lattice.”
A new paper shows that borophene is possible – now it just has to be made!
Zachary A. Piazza, Han-Shi Hu, Wei-Li Li, Ya-Fan Zhao, Jun Li, Lai-Sheng Wang.Planar hexagonal B36 as a potential basis for extended single-atom layer boron sheets. Nature Communications, 2014; 5 DOI: 10.1038/ncomms4113
Brown University Press Release:

Unlocking the secrets of the B36 cluster
A 36-atom cluster of boron, left, arranged as a flat disc with a hexagonal hole in the middle, fits the theoretical requirements for making a one-atom-thick boron sheet, right, a theoretical nanomaterial dubbed “borophene.” Credit: Wang lab/Brown University
Graphene, a sheet of carbon one atom thick, may soon have a new nanomaterial partner. In the lab and on supercomputers, chemical engineers have determined that a unique arrangement of 36 boron atoms in a flat disc with a hexagonal hole in the middle may be the preferred building blocks for “borophene.”
Researchers from Brown University have shown experimentally that a boron-based competitor to graphene is a very real possibility.
Lai-Sheng Wang, professor of chemistry at Brown and his research group, which has studied boron chemistry for many years, have now produced the first experimental evidence that such a structure is possible. In a paper published on January 20 in Nature Communications, Wang and his team showed that a cluster made of 36 boron atoms (B36) forms a symmetrical, one-atom thick disc with a perfect hexagonal hole in the middle.
“It’s beautiful,” Wang said. “It has exact hexagonal symmetry with the hexagonal hole we were looking for. The hole is of real significance here. It suggests that this theoretical calculation about a boron planar structure might be right.”
It may be possible, Wang said, to use B36 basis to form an extended planar boron sheet. In other words, B36 may well be the embryo of a new nanomaterial that Wang and his team have dubbed “borophene.”
“We still only have one unit,” Wang said. “We haven’t made borophene yet, but this work suggests that this structure is more than just a calculation.” ……..
Wang’s experiments showed that the B36 cluster was something special. It had an extremely low electron binding energy compared to other boron clusters. The shape of the cluster’s binding spectrum also suggested that it was a symmetrical structure. ……..
…… That structure also fits the theoretical requirements for making borophene, which is an extremely interesting prospect, Wang said. The boron-boron bond is very strong, nearly as strong as the carbon-carbon bond. So borophene should be very strong. Its electrical properties may be even more interesting. Borophene is predicted to be fully metallic, whereas graphene is a semi-metal. That means borophene might end up being a better conductor than graphene.
“That is,” Wang cautions, “if anyone can make it.”
Abstract: Boron is carbon’s neighbour in the periodic table and has similar valence orbitals. However, boron cannot form graphene-like structures with a honeycomb hexagonal framework because of its electron deficiency. Computational studies suggest that extended boron sheets with partially filled hexagonal holes are stable; however, there has been no experimental evidence for such atom-thin boron nanostructures. Here, we show experimentally and theoretically that B36 is a highly stable quasiplanar boron cluster with a central hexagonal hole, providing the first experimental evidence that single-atom layer boron sheets with hexagonal vacancies are potentially viable. Photoelectron spectroscopy of B36− reveals a relatively simple spectrum, suggesting a symmetric cluster. Global minimum searches for B36− lead to a quasiplanar structure with a central hexagonal hole. Neutral B36 is the smallest boron cluster to have sixfold symmetry and a perfect hexagonal vacancy, and it can be viewed as a potential basis for extended two-dimensional boron sheets.